Difference between revisions of "Mixture Analysis"
(→People) |
(→People) |
||
| Line 16: | Line 16: | ||
* Dave Patterson | * Dave Patterson | ||
* Melanie Schnell @CFEL(http://mpg.cfel.de/asg/sdccm/) | * Melanie Schnell @CFEL(http://mpg.cfel.de/asg/sdccm/) | ||
| − | * Edem Tsikata (Now a | + | * Edem Tsikata (Now a Research Fellow at [http://connects.catalyst.harvard.edu/Profiles/display/Person/121368 Massachusetts Eye and Ear Infirmary]) |
== Publications == | == Publications == | ||
* [[Media:Napthalene_pccp.pdf|Cooling and Collisions of Large Gas Phase Molecules.]] D. Patterson, E. Tsikita, and J.M. Doyle. Phys Chem Chem Phys. 12(33), 9736-41 (2010) | * [[Media:Napthalene_pccp.pdf|Cooling and Collisions of Large Gas Phase Molecules.]] D. Patterson, E. Tsikita, and J.M. Doyle. Phys Chem Chem Phys. 12(33), 9736-41 (2010) | ||
Revision as of 09:45, 15 June 2014
About The Mixture Analysis Experiment
The cooling of large molecules, including biomolecules is a relatively unexplored area of cold molecular physics.
The vast majority of experiments with larger, cold molecules have come from supersonic jets; since it was developed in the late 1970s, these jets have proven to be a versatile workhorse, producing cold samples of thousands of molecular species. Although these samples are cold, the samples are in general moving very rapidly in the lab frame and expanding rapidly. In contrast, buffer gas cooled samples are at or near rest in the lab frame; in addition, if a molecule in a buffer gas is excited, it has a high probability of being 'recycled' back to the ground state via collisions with the buffer gas. These advantages suggest that buffer gas cooled samples provide an attractive and sensitive alternative to supersonic jets for certain classes of spectroscopic studies.
We recently demonstrated that naphthalene, a two ring aromatic hydrocarbon, can be efficiently cooled via buffer gas cooling. The upper bound on the size and complexity of molecules which can be effectively cooled by buffer gas cooling is unknown. We are exploring techniques for cooling these large molecules as well as the collisional physics of large molecules in the gas phase.
A powerful application of buffer gas cooling of large molecules is in broadband, high resolution spectroscopy. UV-visible absorption spectra of large molecules are in general composed of broad, unresolvable manifolds of thousands of ro-vibrational lines. When large molecules are vibrationally and rotationally cooled, their absorption spectra become greatly simplified. If a complex mixture of many such molecules could be efficiently cooled, UV-visible or microwave spectroscopy could effectively resolved, even if the mixture was composed of thousands of a-priori unknown components. Early results suggest that arbitrary mixtures of large molecules can be effectively cooled via buffer gas cooling.
Current research is focused on developing techniques to spectroscopically identify constitutions of a complex mixture. Currently, laser induced fluorescence excited by a widely tunable OPO laser is used to identify features across an arbitrary region of the spectrum. This system provides a flexible testbed for cooling a large number of molecular species and mixtures. Future experimental possibilities include single and double resonance microwave spectroscopy, UV-visible and NIR fourier transform spectroscopy, and integration with gas chromatography to pre-separate the mixture.
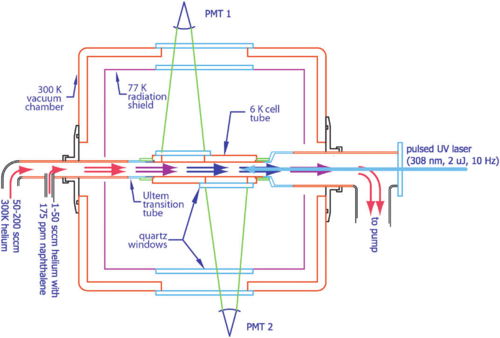
The Set Up
People
- Dave Patterson
- Melanie Schnell @CFEL(http://mpg.cfel.de/asg/sdccm/)
- Edem Tsikata (Now a Research Fellow at Massachusetts Eye and Ear Infirmary)
Publications
- Cooling and Collisions of Large Gas Phase Molecules. D. Patterson, E. Tsikita, and J.M. Doyle. Phys Chem Chem Phys. 12(33), 9736-41 (2010)